Patient
Library
The purpose of our library is to give
you the opportunity to learn some basic yet important
information, presented in a manner that is easy to understand.
The topics presented are directly related to what is
called Holistic Dentistry, which is an approach to dental
treatment by caring for patients' health and safety
from both a conventional as well as "alternative
healthcare" point of view. It is sometimes called
"biological" dentistry or "biocompatible"
dentistry.
Amalgam
Fillings - Silver
Mercury
Fillings - Mercury
Amount
Fillings - Mercury
Toxicity
Mercury Free Fillings
Biocompatible Materials
Amalgam
Amalgam is the generic term applied to a variety of
very similar products used in dentistry to fill teeth.
Amalgam is also known as the  "silver
filling" (due to its shiny appearance) or the silver-mercury
filling. Amalgam literally means "mixed with mercury",
and in the dental sense that is true. Powdered metals
and metal compounds consisting of silver, copper, tin,
and zinc are mixed with about an equal weight of liquid
mercury. Three different types of chemical reactions
take place within this mixture. The resultant amalgam
will set at room temperature, and most importantly,
within a few minutes. "silver
filling" (due to its shiny appearance) or the silver-mercury
filling. Amalgam literally means "mixed with mercury",
and in the dental sense that is true. Powdered metals
and metal compounds consisting of silver, copper, tin,
and zinc are mixed with about an equal weight of liquid
mercury. Three different types of chemical reactions
take place within this mixture. The resultant amalgam
will set at room temperature, and most importantly,
within a few minutes.
Amalgam has been used as a filling
material for 160 years and has enjoyed the reputation
of being an inexpensive, long lasting filling. The materials
alone only cost about one dollar. Although the average
life span of an amalgam filling is only around five
years according to Dr. Leon Silverstone at the University
of Colorado, some amalgam fillings have been known to
last for up to 20 years.
Three times now, amalgam has been accused
of initiating diseases. The first was in the 1830's,
again in the 1920's, and the third time a movement started
in 1973 in which more substantial information became
available to determine the toxicity of the substance.
Up until recently, it was felt that the mercury stayed
within the filling. Now it is known that mercury leaches
out every minute of the day. The average amalgam filling
releases about 34 (plus-minus 2) micrograms of mercury
daily.
Go
to top
Fillings
- Silver Mercury
Mercury comprises about 50% of the most common fillings
in the world called silver-mercury amalgam. Amalgam
also contains copper, tin, silver and zinc. 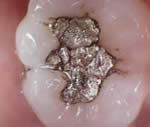 Due
to the high mercury content, it is silver colored when
first placed; therefore, the name, "silver"
filling. After it has been in the mouth, the mercury
begins to react chemically and the resultant corrosion
products are black. The blacker the filling, the more
tarnish has taken place. Fillings have an electrical
current, which can be measured. The higher the current,
the faster mercury is being released. As of 1976, the
American Dental Association (ADA) sponsored (and held
the patent for) high copper amalgam started taking over
the market. Mercury is released 50 times faster from
high copper (around 30%) amalgam than the "conventional"
amalgam of before that time. Due
to the high mercury content, it is silver colored when
first placed; therefore, the name, "silver"
filling. After it has been in the mouth, the mercury
begins to react chemically and the resultant corrosion
products are black. The blacker the filling, the more
tarnish has taken place. Fillings have an electrical
current, which can be measured. The higher the current,
the faster mercury is being released. As of 1976, the
American Dental Association (ADA) sponsored (and held
the patent for) high copper amalgam started taking over
the market. Mercury is released 50 times faster from
high copper (around 30%) amalgam than the "conventional"
amalgam of before that time.
Go
to top
Fillings
- Mercury Amount
How much mercury is in a filling? Currently dental amalgam
fillings contain around 48 to 51% mercury by weight.
Copper comes in second with the high copper amalgam
now leading the markets. Copper can be from 24 to 33%
of the amalgam. The higher the copper level, the faster
both the mercury and copper are emitted from the fillings.
If a gold crown is anywhere in the mouth, mercury comes
off faster. According to university studies done by
Dr. Chew, over the first two years after placement,
amalgams release about 34 micrograms per filling (per
square centimeter of filling exposed) per day. These
tests were done on fillings sitting in pure water and
tested daily.
There are many things that make mercury
come out faster. As just mentioned, any other metals
such as gold crowns, nickel crowns and removable bridges
will increase the speed of release.  Chewing
foods increases the emissions dramatically. Hot liquids,
like coffee, increase the release by thousands of percent,
but only for 10 or 15 minutes. Abrasion from chewing
gum increases the release by 1500% as published by the
ADA. Abrasion during the grinding of teeth during waking
or sleeping hours, called "bruxism," also
releases mercury vapor. Compression of the filling from
chewing releases mercury into the mouth. The electrical
charge on a filling gives a hint as to how fast mercury,
copper and other metals are being released. The higher
the current measured, the faster the mercury release.
The total amount of mercury released would be difficult
to measure, but suffice it to say that the current measurements
are adequate to contribute significantly to disease
processes, and the actual total mercury release in a
living human being with saliva (which has a much higher
electrical potential with dissimilar metals than water)
in a warm mouth with acidic foods, bruxism, chewing
gum, eating foods and several hundred bacterial strains
is greater than any of today's estimates. Chewing
foods increases the emissions dramatically. Hot liquids,
like coffee, increase the release by thousands of percent,
but only for 10 or 15 minutes. Abrasion from chewing
gum increases the release by 1500% as published by the
ADA. Abrasion during the grinding of teeth during waking
or sleeping hours, called "bruxism," also
releases mercury vapor. Compression of the filling from
chewing releases mercury into the mouth. The electrical
charge on a filling gives a hint as to how fast mercury,
copper and other metals are being released. The higher
the current measured, the faster the mercury release.
The total amount of mercury released would be difficult
to measure, but suffice it to say that the current measurements
are adequate to contribute significantly to disease
processes, and the actual total mercury release in a
living human being with saliva (which has a much higher
electrical potential with dissimilar metals than water)
in a warm mouth with acidic foods, bruxism, chewing
gum, eating foods and several hundred bacterial strains
is greater than any of today's estimates.
Go
to top
Fillings
- Mercury Toxicity
Where does all of this mercury go? Into your body. Absorption
of mercury from the area under your tongue and the insides
of your cheeks are the fastest absorption. These areas,
of course, are in close proximity to the fillings, so
efficiency of absorption is great. From these tissues,
the mercury can destroy adjacent tissues, or travel
to the lymphatic drainage system and directly into the
blood stream. From the blood stream, mercury can travel
to any cell in the body, where it can either disable
or destroy the tissues. Mercury can also travel directly
from the fillings into the lungs, into the blood stream,
and, as before described, every cell in the body becomes
a valid target.
 Mercury
and its compounds are adept at traveling through the
"lipid soluble" cell membranes. Cell membranes
contain roughly 60% protein and 40% fat. Nerve cells
are an exception, containing nearly 75% fat. These fat-rich
membranes determine what enters the cell and what does
not. Methyl mercury is oxidized into the "ionic"
form of mercury. This is a very destructive form of
mercury. (Its problem is that it cannot travel very
far.) Methyl mercury is the most dangerous form due
to its ability to travel great distances and enter all
cells. After the trip, it is converted into ionic form.
The ionic form is what actually disrupts internal structures
and metabolic pathways that keep a cell alive and producing
proteins, enzymes, hormones, etc., that are the purpose
of the existence of the cell. Mercury
and its compounds are adept at traveling through the
"lipid soluble" cell membranes. Cell membranes
contain roughly 60% protein and 40% fat. Nerve cells
are an exception, containing nearly 75% fat. These fat-rich
membranes determine what enters the cell and what does
not. Methyl mercury is oxidized into the "ionic"
form of mercury. This is a very destructive form of
mercury. (Its problem is that it cannot travel very
far.) Methyl mercury is the most dangerous form due
to its ability to travel great distances and enter all
cells. After the trip, it is converted into ionic form.
The ionic form is what actually disrupts internal structures
and metabolic pathways that keep a cell alive and producing
proteins, enzymes, hormones, etc., that are the purpose
of the existence of the cell.
All of this travel and destruction
is what defines mercury toxicity. It may favor nerve
tissue for a destruction target, but the kidney is high
up on its hit list of tissues to destroy. After these
two areas, it can wreak havoc in any tissue that might
get in its way. For this reason, it is difficult to
devise a change in the normal chemistry of the body
called a test, which would "prove" mercury
toxicity. It can alter almost anything in the body;
therefore, it should not be allowed to enter the body
for any reason.
Go
to top
Mercury Free
Fillings
In Holistic Dentistry, there is an effort to find biocompatible
materials to reduce the potential for toxicity.  Silver
mercury "amalgam" fillings are avoided due
to toxicity concerns. Amalgam removal and replacement
with natural-looking bonded materials is the standard. Silver
mercury "amalgam" fillings are avoided due
to toxicity concerns. Amalgam removal and replacement
with natural-looking bonded materials is the standard.
Old mercury fillings have cracked and
leaking edges, meaning that liquids and food are going
to invade the internal part of the tooth, creating new
decay. Metal free biological restoration used in holistic
dentistry are providing by far a better marginal seal
that will prevent leaking and also will strengthen the
tooth floor adding fluoride to dental structure. New
esthetic materials are strongly bonded to the tooth.
The resulting restoration restores not just function
and beauty, but returns most of the original strength
of the tooth.
Go
to top
Biocompatible
Materials
Over the years as dentistry has evolved, there have
been a growing number of options available for repairing
teeth. The goal of dentistry has always been to develop
materials that would simulate natural tooth structure
 as
closely as possible, in both appearance and physical
properties, and avoid any potential harmful side effects. as
closely as possible, in both appearance and physical
properties, and avoid any potential harmful side effects.
In addition to concerns of biocompatibility,
when choosing a restoration we have to consider the
size of the defect that is being repaired, the function
of the tooth, and the biting surface involved. Strength,
durability, and cosmetic acceptability are all considerations
when choosing a restorative material.
|